Michelle Dolan, MD1, Nancy J. Mendelsohn, MD2,3, Mary Ella Pierpont, MD, PhD2,3,4, Lisa A. Schimmenti, MD4, Susan A. Berry, MD4, and Betsy Hirsch, PhD1
Objective
Whole genome analysis by array-based comparative genomic hybridization has led to a rapid increase in the discovery of novel microdeletion and/or microduplication syndromes. Here we describe the clinical and cellular genomic correlations of novel microdeletions/microduplications of 19p13.13. METHODS: Among patients referred to the Cytogenetics Institute for array-based comparative genomic hybridization analysis, we identified four with deletions and one with a duplication within 19p13.13. Confirmatory fluorescent in situ hybridization and parental examination were performed. Detailed clinical findings and array profiles were reviewed and compared.
RESULTS: Patients with a deletion of 19p13.13 share a unique set of phenotypic abnormalities. In addition to developmental defects, the microdeletion was associated with overgrowth, macrocephaly, and ophthalmologic and gastrointestinal findings. In contrast, a single microduplication was associated with growth retardation and microcephaly. CONCLUSIONS: Consistent clinical findings associated with copy number variants in this region collectively justify the term 19p13.13 microdeletion/microduplication syndrome. A minimal region of approximately 311-340 Kb of overlap containing 16 genes was identified. Candidate genes include MAST1, NFIX, and CALR. The identification of this syndrome recommends that patients with this copy number variant be subjected to diagnostic close examination and follow-up. Integration of detailed clinical and array data is important to advance both patient care and human genome research.
Genet 2010:12(8):503-511.
Keywords:
Array CGH, chromosome 19p13, microdeletion, microduplication, syndrome
The incorporation of microarray technology into the routine evaluation of patients with unexplained developmental and/or phenotypic abnormalities has resulted in the rapid discovery of numerous repetitive microdeletions/microduplications. For example, in just two years, previously undetected losses and/or gains to the 1q21.11,2 and 16p11.23,4 genomic regions have become widely recognized as potential etiologic factors in autism and related disorders. Clinically significant copy number variants (CNVs) with chromosomal regions too small to be detected by conventional cytogenetic testing have been identified for all chromosome pairs of the human karyotype. For some copy number variants, variation in clinical manifestations and penetrance complicates the establishment of clinical significance. In others, specific phenotypic findings more consistent with copy number variants and association with novel inheritance facilitate demonstration of clinical significance. Here we describe a copy number variant of chromosome 19p13.13 that meets the latter criteria. Despite the fact that chromosome 19 is one of the most gene-rich chromosomes (~2000 genes, 59 Mb), for individuals with deletions involving 19p.5-10, to date, we have only been able to identify this microdeletion/microduplication syndrome with significant deletions by four clinical geneticists The clinical findings and characteristics of the individual reports were nearly identical and their microarray data were correlated.
Materials and Methods
Subject
Five patients were referred to the University of Minnesota Amplatz Children’s Hospital and the Genetics Clinic at Children’s Hospital of Minnesota for diagnosis. Array-CGH was ordered as a component of the standard thorough examination for patients with multiple malformations and/or developmental disorders of unknown cause. Patient ages ranged from 5 to 26 months at the time of initial testing and were evaluated by one of four clinical geneticists each. Participation in the study to allow for data sharing and publication of results was approved by the investigational review committees at both sites. Informed consent was obtained from the parents of these patients.
Array-based comparative genomic hybridization analysis
DNA from peripheral blood of patients was isolated, restriction enzyme digested, and labeled with the fluorescent dye cyanine 5 using random primers and exocrenow fragment DNA polymerase. DNA from pooled sex-matched controls was simultaneously labeled with the fluorescent dye cyanine 3. Patient and control DNA were combined and array-based comparative genomic hybridization (aCGH) was performed using customized 44K oligonucleotide arrays containing target regions of enriched coverage on the backbone with an average of 35 Kb overall median probe interval. To more accurately estimate the stop and start points for the patient with the smallest deletion (patient 3), a 180K array with an average tile spacing of 13 Kb was performed (Agilent Technologies, Santa Clara, CA). The ratio of patients to control DNA for each oligonucleotide was calculated using feature extraction software 9.1 or 10.5 (Agilent Technologies) and analyzed using DNA Analytics 4.0.85 (Agilent Technologies). Statistical algorithms used in the analysis included ADM1 and ADM2
(Agilent Technologies) The threshold is set to an absolute value of 0.3 on a logarithmic scale, with a requirement for three consecutive oligos that meet the threshold criteria.
Table 1 Clinical and cytogenetic characteristics of patients lost or gained within 19p13.12-p13.13
| patient | 1 | 2 | 3 | Four | Five | Patient 8 of Lysy et al. | Patient 9 of Auvin et al. | Patient 10 of Jensen et al. | Patient 7 of Engels et al. | Patient 6 of Stratton et al. |
|---|---|---|---|---|---|---|---|---|---|---|
| cytogenetic imbalance | Deletion 19p13.13-p13.2 | Deletion 19p13.13-p13.2 | Deletion 19p13.13 | Deletion 19p13.13-p13.2 | Duplicate 19p13.12-p13.2 | Deletion 19p13.13-p13.2 | Deletion 19p13.13 | Deletion 19p13.12 | Deletion 19p13.12 | Duplicate 19p13.13-p13.2 |
| Breakpoint (min) | 12,498,237–13,126,508 | 12,536,641–13,794,080 | 12,793,474–13,104,643 | 12,411,017–13,120,904 | 12,601,112–14,488,238 | 10,256,871–13,188,698 | 12,615,927–13,280,259 | 13,838,264–16,357,778 | not listed | No rating |
| Size (min) | 0.6 Mb | 1.3 Mb | 0.3 Mb (311 Kb) | 0.7 Mb | 1.9 Mb | 2.9 Mb | 0.6 Mb | 2.5 Mb | 2.1 Mb | not listed |
| Breakpoint (maximum) | 12,476,664–13,178,511 | 12,521,732–13,854,243 | 12,779,366–13,120,858 | 12,335,402–13,126,464 | 12,582,355–14,503,887 | 10,246,651–13,280,203 | not listed | not listed | not listed | No rating |
| Size(max) | 0.7 Mb | 1.3 Mb | 0.3 Mb (341 Kb) | 0.8 Mb | 1.9 Mb | 3.0 Mb | 0.7 Mb | not listed | not listed | not listed |
| First-stage exam | ||||||||||
| age | 2 years old | 2 years old | 0.75 years old | 0.5 years old | 3 years old | Birth | Normal growth parameters at birth | Birth | Birth | Birth |
| length(%ile) | 50th | >95th | 90th | 90th | <5th | <-2 | SD | not listed | <3rd | 25–50th |
| Weight (%ile) | 75th | >95th | 50–75th | 90th | 25th | <-2 | SD | <1st | not listed | 20th |
| Circumference of preoccipital cortex (%ile) | >97th | >95th | >98th | >98th | <-2 SD | -2 | SD | not listed | <<3rd | 15th |
| craniofacial | Macrocephaly, frontal prominence | Macrocephaly, frontal prominence, and downward cleft palate | Macrocephaly, frontal prominence, and downward cleft palate | Macrocephaly, frontal prominence | Microcephaly, frontal protuberance, and downward cleft palate | Complex craniosynostosis, frontal bulge, small nose, low nasal bridge | Macroceph, high, large forehead, flat philtrum, small mouth | Flat occiput, high forehead, drooping PF, long tongue, short nose | micro/blackie, thin upper lip, long philtrum, small mouth | Microceph, bilateral ridges, upward PF |
| ophthalmology | Esotropia, nystagmus, poor fixation | Optic nerve hypoplasia, exotropia | Optic nerve hypoplasia, exotropia | Optic nerve atrophy, exotropia | Optic nerve hypoplasia, exotropia, nystagmus | Orbital hypoplasia, binophthalmia, proptosis, strabismus | no button | Strabismus, optic disc cupping, ptosis, redundant inner canthus skin | medial canthus redundant skin | Intermittent proptosis |
| G.I. | chronic diarrhea | Abdominal pain, vomiting, poor feeding | none | abdominal pain, vomiting, celiac disease | Abdominal pain, vomiting, FTT (G tube) | not listed | severe constipation | not listed | not listed | Colic after birth |
| brain | normal | Mild atrophy, frontal lobe | Cystic lesion with no rostral corpus callosum | Chiari I malformation with syrinx | normal magnetic resonance imaging | Moderate ventricular enlargement | Conventional magnetic resonance imaging | Mild hypoplasia of the corpus callosum and cerebellar parenchyma, early rupture of membranes 4th vent | usually | Normal head CT |
| seizure | – | – | + | + | + | not listed | + | not listed | not listed | not listed |
| hypotonia | + | – | + | + | + | + | not listed | + | not listed | |
| developmental delay | Full scale IQ 49 | 18 months of skill in 3 years. significant speech delay | mostly nonverbal | Moderate speech delay, special education needs. | 21-29 months of skill in 4.5 years. significant speech delay | It was 33 months. No language in 3.7 years | Saturday in 2 years “Psychomotor retardation” | Full scale IQ 63 | Crawl at 16 months. no speech | Motor milestone not reached, 4 months |
| final exam | ||||||||||
| age | 6 years old | 3 years old | 14.5 years old | 9.5 years old | 7 years old | 3.7 years old | 2 years old | not listed | 1.5 years old | 0.75 years old |
| Elongation (percentile) | 86th | >95th | 50th | 75th | <3rd | -3 SD | >+3 SD | not listed | <3rd | 50th |
| Weight (percentile) | 55th | >95th | 10–25th | 50–75th | 50th | -0.9 SD | +2 SD | not listed | <3rd | 5th |
| Occipitofrontal circumference (percentile) | >97th | >95th | >98th | >98th | -2 SD | -0.7 SD | +2.5 SD | not listed | <<3rd | <5th |
Fluorescence in situ hybrid analysis
Fluorescence in situ hybridization (FISH) was performed to confirm the presence of deletions and/or duplications in the proband and to identify interchromosomal rearrangements in the parents that, if discovered, would have a significant impact on recurrence risk. was excluded. BACs selected for FISH probes were RP11-654K9 (contains MAST1), RP11-963I8 (contains NFIX), RP11-14L14 (contains CACNA1A), and for patient 5 RP11-56K21 (PKN1). (including). Stubs for RP11-654K9, RP11-14L14, and RP11-56K21 were obtained from CHORI BAC/PAC resources, grown on Luria broth plates using chloramphenicol, and nick-translated (Abbott Molecular, Abbott Park, IL). RP11-963I8 prelabeled with Enzo-Green was obtained from Empire Genomics (Empire Genomics, Buffalo, NY). A probe against the 19q subtelomeric region (D19S238E; Abbott Molecular) was also used as a control. Ten metaphase cells were scored to assess the presence or absence of deletions , and 75-100 interphase cells were scored to assess the presence or absence of duplications .
Result
Clinical findings
Detailed medical history and findings for each patient are shown below and summarized in Table 1.
Patient 1
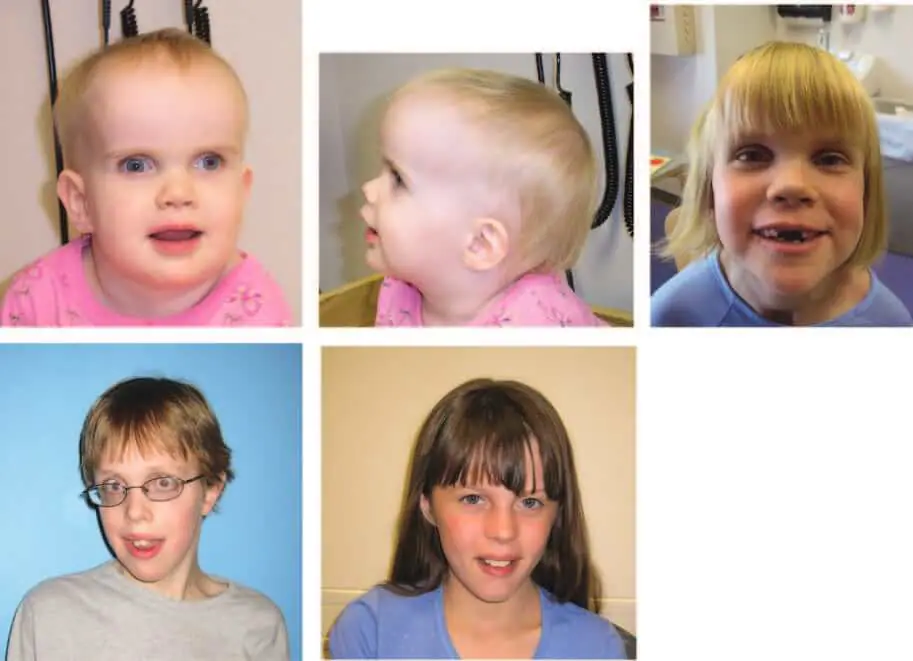
This baby girl was born at term (birth weight 4026 g) (Figure 1). Her neonatal abnormalities included jaundice and an inability to breastfeed requiring amputation of the frenulum of the tongue. Her parents had concerns about her development at three months of age, but it became apparent as she got older, and over time her gross motor movements and her included a language delay. Ophthalmological and visual problems included esotropia, jerky eye movements, end-gaze nystagmus, and poor fixation. The patient had delayed visual acuity that improved over time and underwent two surgeries to correct strabismus. Gastrointestinal disorders characterized by chronic diarrhea of unknown etiology were present for the first 4 years. Physical examination after 22 months showed macrocephaly (>97th percentile), height at the 50th percentile, and weight at the 75th percentile. A prominent frontal protuberance with a supraorbital bulge and deeply set eyes was noted, along with a depressed nasal bridge, an upturned nasal tip, and a small mouth with a relatively high arched palate. Her fingers and toes were long, and there were deep wrinkles on the soles of her feet. The patient was hypotonic and had good deep tendon reflexes. At examination at 30 months, her height and weight were above the 80th percentile, and her occipitofrontal circumference was >97th percentile, but at 22 months she was only 0.3 cm larger. Follow-up at 6 years of age showed height in the 86th percentile, weight in the 55th percentile, and preoccipital circumference 1 SD above the 97th percentile. In Wechsler’s most recent evaluation, her preschool battery verbal IQ was 61, performance IQ was 47, and full scale IQ was 49. Her fine motor and visual motor skills have been repeatedly assessed and are relatively delayed. Radiological examination of her cranial and bone age was normal with X-ray and CT. Magnetic resonance imaging (MRI) of the brain showed mild hypoplasia of the prechiasmatic optic nerve, but the brain was structurally normal
It was. Echocardiography and renal ultrasound were normal. Laboratory tests showed a normal 46,XX karyotype (550 band level resolution), normal urine metabolic screen, and normal serum amino acids, ammonia, lactate, and pyruvate.
Patient 2
This baby girl was born at term (birth weight 2835 g). After 6 months, she was noted to have delayed gross and fine motor development, and she was unable to visually focus and track. Her patient was macrocephalic with a preoccipital circumference of 46.5 cm (95th percentile) and had a downward-pointing palpebral fissure. The Bayley Infant Development Test for infant development at 7 months of age was 3 months, gross motor level was 2 to 5 months, and fine motor level was 1 month. After 15 months, her brain waves were normal and her hearing was normal. At 17 months, the patient presented with chronic and transient abdominal pain associated with vomiting. Magnetic resonance imaging of the brain showed mild atrophy, particularly in the frontal lobes. Exotropia persisted, and ophthalmological examination showed bilateral optic nerve hypoplasia. Heart and renal sounds were normal. Cytogenetic analysis showed a normal 46,XX karyotype. The clinical findings suggested Sotos syndrome, so NSD1 was sequenced and the results were negative. there were. At follow-up evaluation, language and developmental delays persisted, with a proficiency level of approximately 18 months at age 3 years, significant language delay (apraxia of language), and preoccipital circumference persisted above the 95th percentile. He was shown to have sexual macrocephaly.
Patient 3
This boy was born with a birth weight of 3884 g and presented with large macrocephaly in infancy, a wide forehead, a frontal prominence, an inferior oblique palpebral fissure, hypotonia, and marked developmental delay. (Figure 1). The patient developed epilepsy at the age of 4 years and has been medically controlled. He underwent two surgeries to correct strabismus and upper eyelid retraction (sunken eye sockets and decreased blinking). From infancy to age 14, his macrocephaly persisted (preoccipital circumference >98th percentile), but height gradually increased from the 90th to the 50th percentile and weight from the 50th to 75th percentile to the 10th to 25th percentile. It declined to . Ophthalmological follow-up revealed optic atrophy with onset between the ages of 8 years (normal optic nerve by magnetic resonance imaging) and 11 years (retrobulbar, prechiasmatic, and chiasmatic optic nerve hypoplasia). At the most recent examination (age 14.5 years), he was in the 25th to 50th percentile for height, 10th to 25th percentile for weight, and 98th percentile for head circumference. Characteristics of transformation
They had low facial tone, large ears, a high palate, crowded teeth, large hands, and flat feet. He had severe speech delay and was nonverbal except for a few selected words. Magnetic resonance imaging of his brain showed a chronic occipital cystic lesion of unknown origin and a rostral absence of the corpus callosum. Serial renal ultrasound revealed left renal pelvis dilatation or bulge, interpreted as extrapelvic, which remained stable. Cytogenetic testing showed a normal 46 XY karyotype (550 band level resolution), and molecular testing showed deletions or mutations were negative.
Patient 4
This baby girl was born at term (birth weight 4097 g) and exhibited hypotonia, macrocephaly, prominent eyes with large blue irises, and exotropia during infancy, and optic nerve pallor was noted within 1 year ( Figure 1). Other clinical findings include an upturned nose, low facial tone, six crescent-shaped cafe-au-lait spots, and myoclonic jerks, once at age 9 years. The patient developed a prolonged seizure. EEG did not detect epilepsy. She had motor and speech delays including gait at 2-3 years and nonverbal dysarthria 2 years ago. In her fourth year she underwent strabismus surgery, but her early visual inattention improved over time. She developed symptoms of celiac disease between 7 and 9 years and was on a gluten-free diet. At her most recent visit at age 9.5, she requires special education services, but her vocabulary has expanded significantly. Magnetic resonance imaging of her brain after 5 months was normal, but repeat examination 4 years later showed Chiari type I malformation (syringomyelia, surgically decompressed at age 5 years) and chiasm and intracranial lesions. Optic nerve hypoplasia was demonstrated. Laboratory tests include metabolic testing (serum amino acids, blood long chain fatty acids, urine organic acids, muscle biopsy, and mitochondrial DNA testing), cytogenetic testing (750 band level resolution), and NF1 mutation analysis. The results were normal.
Patient 5
This boy was born at 36 weeks gestation (birth weight 2835 g). Two months later, he developed anorexia, constipation, frequent vomiting, marked irritability, multiple infections, and a paler complexion than his siblings. After 14 months, his weight, height, and preoccipital circumference were all below the 5th percentile. Other clinical findings included a tilted forehead, narrow nasal wings, inverted nipples, and abnormal fat distribution in the buttocks. The patient had epilepsy, with approximately 4 to 5 seizures occurring daily from a focus in the left frontotemporal lobe. Because of continued difficulty in feeding and aversion to oral intake, Nissen fundoplication with G-tube placement was performed. Ophthalmological examination revealed horizontal nystagmus. By 3 years, his head circumference remained significantly below the 5th percentile, with persistent growth failure and developmental delay (only a few words, walking at 2 years). Neuropsychological testing at age 4.5 years showed development comparable to ages 21 to 29 months, with hyperactivity, sleep disturbances (delayed sleep onset with approximately 3 hours of sleep per night), tantrums, obsessive-compulsive behaviors, and Self-injurious behavior was observed. His receptive language was mildly impaired and expressive communication significantly impaired, and he first spoke in sentences at age 6. There has been some improvement over the past 2-3 years. The patient’s growth rate is slow (3.2 cm/year, < 1st percentile) and his height at age 7 years is currently below the 3rd percentile, but his weight has increased to the 50th percentile as his oral feeding aversion improves. did. His family history was significant in that the patient’s father had nystagmus and reported learning difficulties. Radiological examinations included chest X-ray, abdominal ultrasound, upper gastrointestinal tract, flexible sigmoidoscopy, and brain magnetic resonance imaging, all of which were normal. Laboratory tests including electrolytes, complete blood count with differential, liver and thyroid function tests, immunoglobulins, sweat chloride, serum amino acids, and urinary organic acids were all reported as normal. Cytogenetic analysis showed a normal 46,XY karyotype with 850 band level resolution. FISH testing (fluorescence in situ hybridization) is used to detect Williams syndrome, telomere FISH, and external BAC aCGH performed at the reference laboratory was normal.
Array-CGH analysis
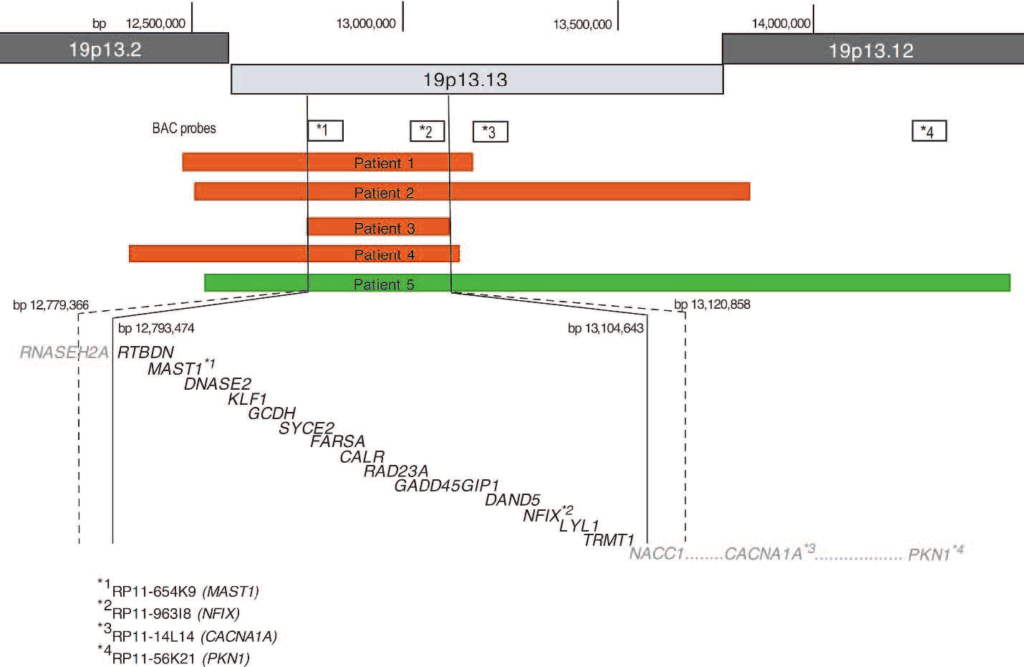
Array-CGH showed ratio profiles matching the loss areas of patients 1 to 4, and the gain area of patient 5. As shown in Figure 2, the area of loss ranged from approximately 0.31 Mb (patient 3) to 1.3 Mb (patient 2). Patient 5’s gain area was ~1.9 Mb. The minimal region of overlap (SRO) separated by the deletions seen in patient 3 is ~ It spans 311 Kb and is localized to band 19p13.13. Based on Human Genome Build 18, the start and stop points of this SRO were 12793474 and 13104643 (minimum breakpoint) and 1279366 and 13120858 (maximum breakpoint). Parents of all patients were followed up with aCGH (Figure 3). The ratio profile of the 19p13 region was within normal range for each of the parental specimens.
Figure 2. Four microdeletions patients ( Patient 1-4) and Microduplication Array comparative genomic hybridization (aCGH) profile of the patient (patient 5). The array plots for patients 1-4 show a range of 0.3Mb minimum (patient 3) to maximum 1.3Mb (patient 2).deletion It shows. The array plot for patient 5 shows 1.8-1.9 Mb of duplication.
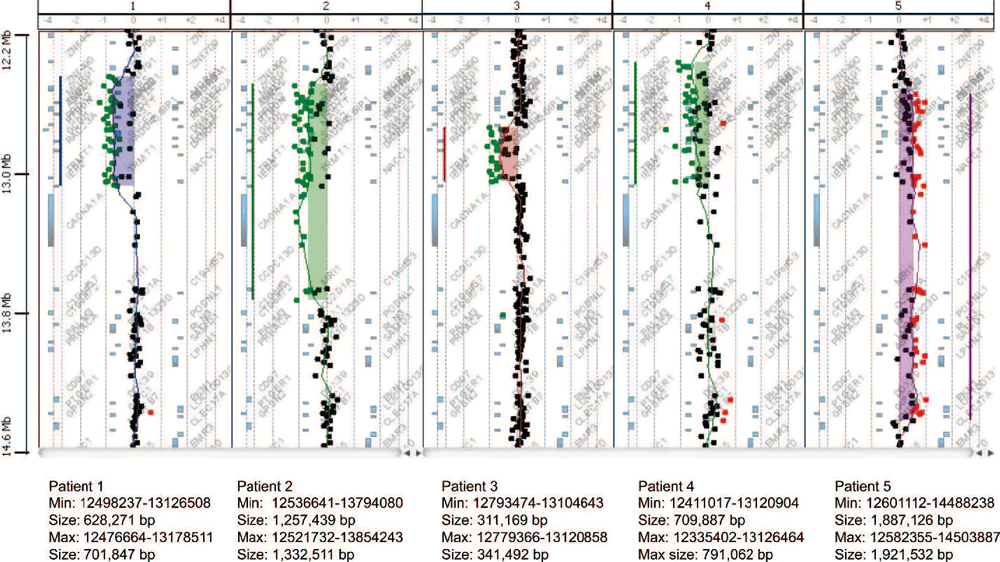
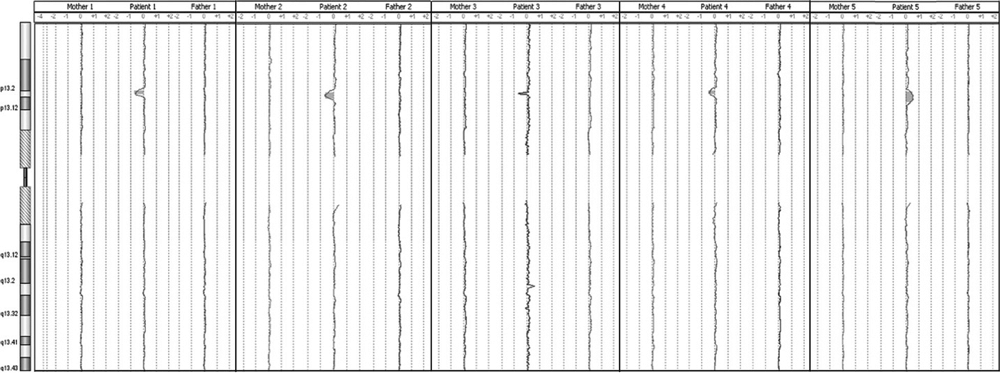
Figure 3. Array comparative genomic hybridization (aCGH) profiles obtained from each patient’s parents (patient array in the center for reference) show no imbalance in regions of interest .
FISH analysis
Copy number loss in patients 1-4 is interstitial deletion Confirmed by FISH as representing Consistent with aCGH results, all cases showed deletion of both RP11-654K9 (Figure 4A) and RP11-963I8, but only patient 2 showed deletion of RP11-14L14 . Patient 5 with increased copy number duplication showed an interphase signal pattern consistent with . For probes RP11-654K9, RP11-963I8, RP11-14L14 and RP11-56K21, 88%, 78%, 80% and 65% of interphase cells showed three hybridization signals, respectively (Figure 4B ). Although replication could not be visualized by FISH on metaphase chromosomes, the signal was only present on chromosome 19, ruling out the possibility that the extra signal was inserted elsewhere in the genome. In the parent FISH test,deletionorduplicationNo evidence was shown, further confirming that all BAC signals were localized to chromosome #19, ruling out the possibility of an interchromosomal insertion (Figure 4C). Therefore, the proband’s losses and gains represent new events. Genes included in SRO: The SRO separated by the deletion region discovered in patient 3 The SRO separated by the deletion region discovered in patient 3 contains 16 genes from RNASEH2A (telomeric) to NACC1 (centromeric). Contains all or part of the genes, including most of RNASEH2, most of RNASEH2, all of RTBDN, MAST1, DNASE2, KLF1, GCDH, syce2, farsa, calr, rad23a, gadd45gip1, dand5, nfix, LYL1 and TRMT1, and NACC1. The majority (Figure 5) (http://genome.ucsc.edu; UCSC hg18 Mar 2006). 11,12
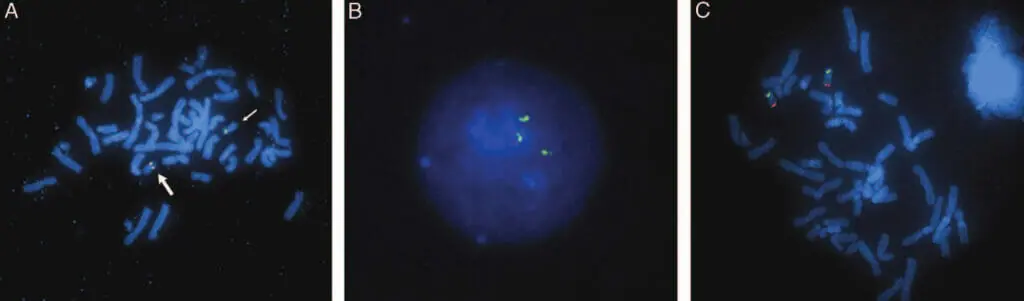
Figure 4. A. FISH was performed using metaphase cells from patient 3 in combination with BAC clones (RP11-654K9, red, RP11-14L14, green). Normal chromosome 19 (large arrow) hybridized to both probes, giving a yellow fusion signal. deleted chromosome 19 (small arrow) hybridizes only to RP11-14L14, There was no red signal. B. Interphase cells from patient 5 show three signals for the RP11-56K21 probe. C. Metaphase cells of the parent specimen showing normal signal pattern using probes RP11-963I8 (green) and D19S238E (red). No interchromosomal rearrangements were observed.
Inspection
aCGH identified 4 patients with microdeletions and microduplications involving 19p13.12-19p13.2 . SRO was delimited by patient 3 with a minimal deletion of approximately 311 Kb (maximum 340 Kb) within 19p13.13 . The smallest SRO ranges from approximately 12793474bp to 13104643bp (Build 18). To date, there are only two published case reports of patients with deletions encompassing this SRO (the patient reported by Auvin et al. 9 ). One case was reported by Lysy et al . 8 with a deletion of approximately 740 Kb . with a larger 3 Mb deletion . Patients in this study and in the study of Auvin et al . show consistent phenotypic correlations supporting the designation of this 19p13.13 clinical-cytogenomic entity as a microdeletion/microduplication syndrome . Lysy et al.’s patient 8 , however, the phenotype was significant, as this deletion affected the telomeres in the present report by nearly 3 Mb, nearly three times the size of the largest deletion and nearly 10 times the size of the critical region.
Figure 5. deletion region in patients 1-5 (red) and the location of the overlapping area (green). The solid and dashed lines indicate the minimum and maximum breakpoints of SRO, respectively. Also shown are the BAC probes (*1–*4) used to confirm the imbalance regions, the genes to which they are mapped, and the genes included in the SRO.
19p13.13deletionsThe clinical findings in patients are a cluster of three recurrent abnormalities. It’s worth noting. First, there is overgrowth. Patients 1-4 and Auvin et al.’s patient 9 Both of these patients were macrocephalic for their age, and their height and weight were consistent with their craniofacial dimensions. Of particular interest are patients 2 and 3 and patient 9 of Auvin et al. To exclude Sotos syndrome, NSD1 testing was performed. Therefore, this region of 19p13.13 has a differential diagnosis of Sotos syndrome, but NSD1 It may be related to other patients with negative test results. The second most common clinical manifestations are ophthalmological abnormalities (particularly strabismus) and optic nerve atrophy or hypoplasia, the latter detected by formal ophthalmological examination and/or magnetic resonance imaging. A third finding is gastrointestinal symptoms, particularly abdominal pain and vomiting. Although the only finding in this triad is not specific to 19p13.13 abnormalities, this set of findings is similar to other Microdeletion/Microduplication This is not one of the many reports currently reported. However, these 19p13.13 patients also showed some nonspecific findings. Neurological abnormalities (eg, seizures, myoclonic spasms, hypotonia) were present in some, and developmental and/or mental retardation was present in all. For comparison, within 19p13.12-19p13.2 microdeletions or A few other published patients with microduplication are included in Table 1 (Reference (See also literature). 6, 7, 10. None of these overlap with the critical region delimited by patient 3, and their phenotype There are some notable differences. Our deletion patient and Auvin et al.’s deletion in contrast to patient 9 these duplication No deletion Patient7,10 are small with age, supporting the conclusion that genes within the 310 Kb SRO that we defined are responsible for the large body size. Our deletion patients do not have hearing loss, but these Do not duplicate19pMissed There is significant hearing loss for aphrodisiacs, suggesting that genes within these proximal and distal regions explain this aspect of their phenotype. However, some similarities exist (e.g. strabismus and optic disc cupping).
Duplication was observed in only one case, and caution should be taken in generalizing the clinical symptoms. Currently well-established reciprocals, including 17p11.2 and 22q11.2 Microdeletion/microduplication syndrome Now, each deletion A set of phenotypic findings associated with Duplication are sufficiently different from those associated with 17p11.2 deletion about Smith-Magenis syndrome、Duplicate of 17p11.2 about Potocki-Lupski syndrome is clearly justified.
Additionally, patients with duplications may have findings opposite to those seen in patients with deletions, as seen with disequilibrium involving the Williams-Beuren locus . 13,14 In this series, this is similarly demonstrated by the striking difference in head circumference between macrocephalic 19p13.13 deletion patients and microcephalic duplication patients. This was also true for the duplicate patients reported by Stratton et al . However, deletions and duplications in the same region may also share some common features, as demonstrated by the finding of velopharyngeal dysfunction in both 22q11.2 deletions and duplications . Similarly, several clinical findings (e.g., ocular findings, gastrointestinal symptoms, and seizures) were observed in 19p cases, regardless of the presence or absence of deletions or duplications .15-17
Mouse models and mutation screens are proposed to further investigate potential candidate genes MAST1 and CALR. As previously mentioned, the start and stop points involved in deletions and duplications were unique to each patient. This fact, coupled with the absence of low-copy repeats or segmental duplications within or adjacent to the deletion/duplication region, makes them unlikely mechanisms for generating 19p13 copy number variants . Opposes allelic homologous recombination. Recently, it has been suggested that microhomology at breakpoint junctions involved in strand break repair is involved in the formation of copy number variants with non-repetitive breakpoints. 32,33 However, detailed sequencing of the breakpoint will be required to further explore this possibility and further characterize the underlying molecular mechanism. The Center for Applied Genomics Research (TCAG) (http://projects.tcag.ca/variation/) has a database of 34 genomic variants (DGV) that are well-documented in cytogeneticists and control populations to identify benign variants. will be invaluable to other researchers working with arrays to help identify copy number variants that may be Note that in DGV, part of this region (from the starting point to 12919491) was identified by Wong et al . Based on the BAC array, it was removed in 3 out of 95 patients. Notably, among the large control cohorts published in the literature and cited in the DGV (representing over 4000 control individuals) , only one identified deletions or duplications of a large proportion of this region. No way. Furthermore, as the deletion and duplication in our case were proven to be de novo, the single 2007 accession in DGV outweighs the data supporting the clinical importance of this copy number variant. isn’t it. Validating the copy number variant breakpoints reported in the 2007 BAC array may also provide further insight.
In summary, close collaboration between clinical geneticists and cytogeneticists, and correlation of genotype and phenotypic data, led to the identification of microdeletion/microduplication syndrome in 19p13.13. made it easier. This leads to a diagnostic workup on identified patients (e.g., a complete ophthalmological examination to detect strabismus and a thorough evaluation including magnetic resonance imaging of the optic nerve, magnetic resonance imaging to detect structural brain abnormalities). Resonance imaging, continuous monitoring of the occipitofrontal area, elucidation of the history of gastrointestinal symptoms including poor feeding, abdominal pain, and vomiting), as well as research to elucidate the relationship with further functions and clinical features of this syndrome. This led to the identification of the gene.
Acknowledgments
The authors would like to thank the patients and their families who actively participated in this study. We also thank Heather Zierhut, MS, Genetic Counselor, for her support of this project, and the technicians at the Cytogenetics Laboratory at Fairview, University of Minnesota Medical Center, for their assistance in performing the array-CGH assay.
References
- Mefford HC, Sharp AJ, Baker C, et al. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med 2008;359:1685-1699.
- Brunetti-Pierri N, Berg JS, Scaglia F, et al. Recurrent 1q21.1 deletions and duplications associated with microcephaly or macrocephaly, developmental and behavioral abnormalities. Nat Genet 2008;40:1466-1471.
- Weiss LA, Shen Y, Korn JM, et al; Autism Consortium. Association of microdeletions and microduplications in 16p11.2 with autism N Engl J Med 2008;358:667-675.
- Marshall CR, Noor A, Vincent JB, et al. Structural chromosomal variations in autism spectrum disorders. Am J Hum Genet 2008;82:477– 488.
- Hurgoiu V, Suciu S. Appearance of 19p-. Ann Genet 1984;27:56-57.
- Stratton RF, DuPont BR, Olsen AS, Fertitta A, Hoyer M, Moore CM. Stromal duplication 19p. Am J Med Genet 1995;57:562–564.
- Engels H, Brockschmidt A, Hoischen A, et al. DNA microarray analysis identifies candidate regions and genes for unexplained mental retardation. Neurology 2007;68:743-750.
- Lysy PA, Ravoet M, Wustefeld S, et al. A new case of symptomatic cranial syndrome with subclinical 19p13.2-p13.13 deletion. Am J Med Genet A 2009; 149A:2564–2568.
- Auvin S, Holder-Espinasse M, Lamblin M-D. Detection of a novel 0.7-Mb deletion in 19p13.13 by Array-CGH, including CACNA1A associated with mental retardation and epilepsy with infantile seizures. Epilepsy 2009;50:2501-2502.
- Jensen DR, Martin DM, Gebarski S, et al. Novel chromosome 19p13.12 deletion in children with multiple congenital anomalies. Am J Med Genet A 2009;149A:396–402.
- Kent WJ, Sugnet CW, Furey TS, et al. UCSC human genome browser. Genome Research 2002;12:996-1006.
- Rhead B, Karolchik D, Kuhn RM, et al. UCSC Genome Browser database: Update 2010 Nucleic Acid Res 2010;38(database publication):D613-D619.
- Kriek M, White SJ, Szuhai K, et al. Copy number variation in adjacent (or non-embedded) regions due to duplication among patients with developmental delay and/or congenital malformations; reciprocal and Detection of partial Williams-Beuren duplications. Eur J Hum Genet 2006;14:180 –189.
- Somerville MJ, Mervis CB, Young EJ, et al. Severe expressive language delay associated with duplication of the Williams-Beuren locus. N Engl J Med 2005;353:1694 -1701.
- Potocki L, Chen KS, Park SS, et al. Molecular mechanism of duplication and reciprocal homologous recombination of the 17p11.2-Smith-Magenis microdeletion. Nat Genet 2000;24:84-87.
- Ou Z, Berg JS, Yonath H, et al. 22q11.2 microduplications are often inherited and associated with a variety of phenotypes. Genet Med 2008;10:267-277.
- Ensenauer RE, Adeyinka A, Flynn HC, et al. Clinical, cytogenetic, and molecular analysis of 3 patients with the emerging syndrome microduplication 22q11.2:1. Am J Hum Genet 2003;73:1027–1040.
- Kawane K, Fukuyama H, Kondoh G, et al. Requirement of DNase II for definitive erythropoiesis in mouse fetal liver. Science 2001;292:1546-1549.
- Basu P, Lung TK, Lemsaddek W, et al. EKLF and KLF2 have compensatory roles in embryonic β-globin gene expression and primitive erythropoiesis. Blood 2007;110:3417-3425.
- Hodge D, Coghill E, Keys J, et al. Global role of EKLF in definitive and primitive erythropoiesis. Blood 2006;107:3359-3370.
- Kolker S, Garbade SF, Greenberg CR, et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatric Research 2006;59:840-847.
- Zschocke J, Quak E, Guldberg P, Hoffmann GF. Mutation analysis in glutaric aciduria type I. J Med Genet 2000;37:177-181.
- Baskett C, Merinero B, Christensen E, et al. Glutaryl-CoA dehydrogenase deficiency in Spain: Evidence from two genetically and biochemically distinct groups of patients. Pediatric Research 2000;48:315-322.
- Goodman SI, Stein DE, Schlesinger S, et al. Glutaryl-CoA dehydrogenase mutations in glutaric acidemia (type I): review and report of 30 novel mutations. Hum Mutat 1998;12:141-144.
- Garland P, Qulaishe S, French P, O’Connor V. Expression of the MAST family of serine/threonine kinases Brain Resin 2008;1195:12-19.
- Borurgeron T. A. From Synaptic Trek to Autism. Curr Opin Neurobiol 2009;19:231– 234.
- Varga EA, Pastore M, Prior T, Herman GE, McBride KL. Prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorder, developmental delay, and macrocephaly. Genet Med 2009;11:111-117.
- Valiente M, Andres-Pons A, Gomar B, et al. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J Biol Chem 2005;32:28936-28943.
- Campbell CE, Piper M, Plachez C, et al. The transcription factor Nfix is essential for normal brain development. BMC Dev Biol 2008;8:52.
- Driller K, Pagenstecher A, Uhl M, et al. Nuclear factor IX deficiency causes brain malformations and severe skeletal defects. Mol Cell Biol 2007;27: 3855–3867.
- Basel-Vanagaite L, Attia R, Yahav M, et al. CC2D1A is a member of a new gene family with a C2 domain and is involved in autosomal recessive nonsyndromic mental retardation. J Med Genet 2006;43:203-210.
- Vissers LELM, Bhatt SS, Janssen IM, et al. Rare pathogenic microdeletions and tandem duplications are stimulated by local genomic structure through microhomology. Hum Mol Genet 2009;18:3579 –3593.
- PJ, Ira G, Rupsky JR, Hastings A microhomology-mediated cleavage-induced replication model for the origin of human copy number variation. PLoS Genet 2009;5:e1000327.
- Iafrate AJ, Feuk L, Rivera MN, et al. Detecting large-scale variations in the human genome Nat Genet 2004;36:949-951.
- Wong KK, deLeeuw RJ, Dosanjh NS, et al. Comprehensive analysis of common copy number variations in the human genome. Am J Hum Genet 2007;80:91–104.
- Redon R, Ishikawa S, Fitch KR, et al. Global variation in copy number in the human genome. Nature 2006;444:444-454.
- Pinto D, Marshall C, Feuk L, Scherer SW. Copy number variation in a control population cohort. Hum Mol Genet 2007;16:R168–R173.
- Zogopoulos G, Ha KCH, Naqib F, et al. Frequency of variation in germline DNA copy number in a large North American population Hum Genet 2007;122:345-353.
- Perry GH, Ben-Dor A, Tsalenko A, et al. Fine scale and complex architecture of human copy number variation. Am J Hum Genet 2008;82: 685–695.
- Shaikh TH, Gai X, Perin JC, et al. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Research 2009;19:1682-1690.
 中文
中文