Hiro Clinic NIPT, together with its affiliated Tokyo Sanitary Laboratory, offers pregnant women what only Hiro Clinic can provide.
The Tokyo Sanitary Inspection Laboratory carries out inspections under technical transfer from two inspection companies.
1.Illumina Corporation (USA)
2.Medicover (Republic of Cyprus)
Both inspections are carried out by the Tokyo Sanitary Laboratory in Itabashi.
Illumina’s test reads the entire genetic region, making it possible to test for aneuploidy (trisomy and monosomy) of all chromosomes and partial deletions and partial duplications of all chromosomes, in addition to the usual sex chromosomes and trisomy 21, 18 and 13. The test is also characterised by relatively rapid results.
The Medicover formula, on the other hand, can read in depth in parts, but only in a limited area. It can detect sex chromosome aneuploidy and chromosome 21, 18 and 13 trisomy, but cannot read all chromosomes. On the other hand, four types of microdeletion syndromes can be detected. Only the Tokyo Sanitary Laboratory can detect microdeletion syndromes in Japan. Of these, Di George syndrome is the most frequent, followed by Down syndrome. The test also takes longer than the Illumina method. It takes a minimum of three days. The good thing about this test is that it can identify Vanishing Twin and even surrogate mother cases. This is because the genetic differences between mother and child can be recognised.
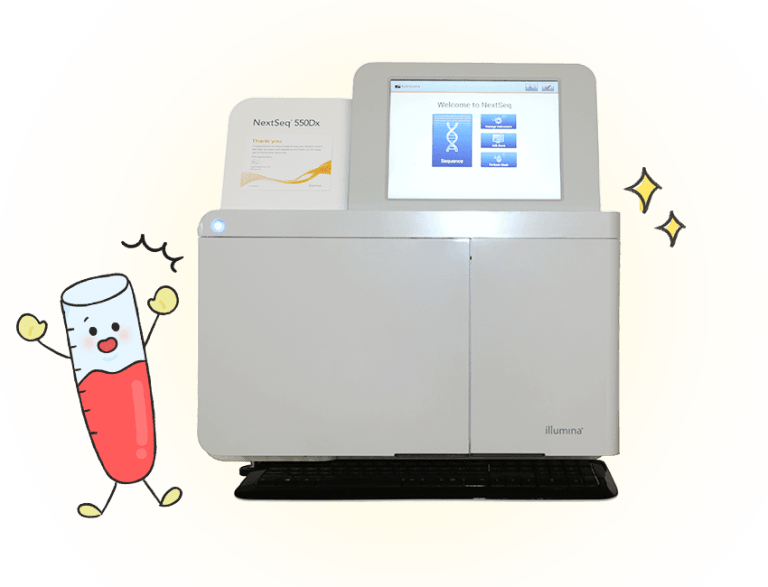
Whichever method is used, the Illumina Nextseq 550DX is used for sequencing. What is different, then, is the extraction of the genes and the location of the markers. Therefore, the testing methods are different.
Hiro Clinic is the only laboratory in Japan that offers these tests in the same group. Moreover, the wide range of variations allows us to cater for a variety of pregnant women.
Hiro Clinic provides a positivity score that calculates not only negative and positive results from data calculated by the Tokyo Sanitary Laboratory (data from the genetic test), but also how accurate the positivity is when it is positive. This service is provided free of charge. In the unlikely event that a pregnant woman tests positive, we consider this to be a reference value for the amniotic fluid test.




Amniotic fluid genetic testing
Exome analysis is performed free of charge if requested by your obstetrician/gynecologist when you have an amniotic fluid test. Please contact Hiro Clinic for more information and we will explain the procedure to you. You will need to take amniotic fluid and send it to the Tokyo Sanitary Laboratory. If you are on amniotic fluid testing support, you can receive a subsidy of up to JPY 200,000 even if you have it done at another clinic. (as of May 2023)
Inspection items
Detection of the presence or absence of trisomy, detection of microdeletion syndromes
Advantages
Unlike the G-band method, detection is possible without the need for cell culture
First in the country! Non-accredited facilities can also be inspected in-country
In ‘certified facilities※1‘, that carry out NIPT testing, all testing is completed domestically. So why do ‘non-certified facilities’ transport the tests abroad ※2?
In pursuing this question, we were confronted with the fact that the way in which certified facilities are in the country is evolving. At Hiro Clinic NIPT, we have accepted this fact and have successfully attempted to introduce further ‘technology’ and new ‘services’. It is now possible to offer the same domestic testing (technology) with better content (services) as the certified facilities!
※1 Facilities accredited by the Japanese Medical Association ※2 When specimens, including blood tests, are sent abroad, the Medical Care Act stipulates that they must pass through facilities that meet the standards specified by the Ministry of Health, Labour and Welfare (Article 15-3(1) of the Medical Care Act)

Results reported in as little as two days! (Illumina method only)
As the tests are carried out in domestic laboratories, the time taken to receive the results(Minimum2days )is reduced compared to conventional testing, where specimens have to be transported to an overseas laboratory.


Over 68,000 cases!(This is the number of cases conducted by the Tokyo Sanitary Inspection Centre only)
We have been chosen by our customers to provide more than 68,000 cases. We have compiled data and statistical figures on these results, which we can pass on to patients who visit us in the future.

Information Technology(IT) and genetic testing
Hiro Clinic uses its own system to manage everything from test applications to barcode issuance and test result reporting. The system aims to reduce input errors prior to testing, and barcodes for blood sampling are linked to domestic tests to avoid mistakes. In addition to the automatic generation of test result PDFs, the statistical data from which previous test results are derived are updated on a daily basis. Amniotic fluid test results are also obtained from patients using the amniotic fluid test support to control the accuracy of the NIPT test.

Hiro Clinic own data (positive scores)
Hiro Clinic provides a positivity score, which indicates how positive the test is likely to be if 21, 18 or 13 trisomy is positive. We provide an easy-to-understand graph showing this number and the related data from previous amniotic fluid test results. Even in the event of a positive result, this provides helpful data to help you react calmly. Positive scores are provided free of charge.

Hiro Clinic is the only clinic in Japan where microdeletion syndrome can be investigated.
Including all certified and non-certified facilities, Hiro Clinic is currently the only clinic in Japan with this technology. The detection area is narrow (around 1 Mbp), making it technically difficult.

You have the right to know.
We can test for total chromosome aneuploidy (which also reveals gender) and total autosomal whole-region partial deletion disease, which is not possible in certified centres. We want to provide as much as we can know. It is often possible in Japan to receive up-to-date medical care after birth. We believe that it is unreasonable to not be able to make a choice before birth.

Comparison of inspections between Hiro Clinic, certified and non-certified facilities
| Hiro Clinic | Japan Medical Association Non-certified medical institution | Japan Medical Association Certified medical institution | |
|---|---|---|---|
| Age limits for pregnant women | None | None | None (Basically 35 over) |
| Inspection items | All chromosomes, chromosomes 1-22, sex chromosomes Whole autosomal whole-region partial deletion disease | All chromosomes, chromosomes 1-22, sex chromosomes Whole autosomal whole-region partial deletion disease | Chromosomes 13, 18 and 21 |
| Inspection body | Domestic Tokyo Sanitary Inspection Institute | Overseas | Basically, domestic |
| Couple together | Unnecessary | Unnecessary | Necessary |
| Number of visits | 1 time | 1 time | More than 3 times |
| Fees | Cheap | Expensive | Expensive |
| Inspection speed | Usually 2-5 days after blood collection※1,2. Please note that the express delivery option is available at all Hiro Clinic NIPT clinics (delivery within 2~3 days of blood collection), except for the collaborating facilities. ※1 Usually within 3-6 days for some clinics and collaborating institutions ※2 Excluding some plans | Fast to normal | Late |
Difference① Testable age
The main difference between facilities certified by the JSME and non-certified facilities is the existence or non-existence of an age limit for pregnant women who can undergo testing. In certified facilities of the Japanese Medical Association, the age of pregnant women is 35 years or older (on the expected date of delivery), except under certain conditions, such as if the woman has had chromosomal abnormalities in previous pregnancies or deliveries.
This means that at certified facilities, NIPT testing is not available for those under 35 years of age, even if they want to undergo the test, especially if no other conditions apply. (As of May 2023, testing is still available for those under 35, but they are not willing to take it.)
Difference② Inspection item
Another thing is that certified centres do not allow testing for anything other than chromosome 13, 18 and 21 disease. This means that even if there is a chromosomal abnormality, a negative test for chromosomes 13, 18 or 21 will result in a negative result. However, with current NIPT technology, it is possible to test for diseases caused by chromosomal abnormalities such as Down syndrome, which can be detected by testing for chromosomes 13, 18 and 21, as well as other chromosomes and diseases caused by partial chromosomal abnormalities such as whole-autosomal partial deletion diseases. Microdeletion syndromes are also now detectable.
The NIPT test also reveals the sex of the baby (in the case of twins, only whether the foetus has a Y chromosome or not), but for the same reason the pregnant woman is not informed about this.
What is CAP?
The Tokyo Health Laboratory, with which Hiro Clinic NIPT is affiliated, was accredited by the College of American Pathologists (CAP) in April 2022.
CAP, which stands for College of American Pathologists, is a third-party certification body initiated by the American College of Pathologists.
Only third-party organisations that have verified and certified the integrity, robustness and validity of their tests are awarded accreditation.
The certification is renewed every two years, and CAP auditors from all over the world visit sanitary laboratories to inspect whether inspections are carried out in accordance with the rules of the certifying body. The survey verifies that the management and operation of the equipment and personnel is carried out correctly, and that errors are verified and reported and corrective actions are taken when they occur. Renewal of this certification must be supervised by a laboratory director who has performed administrative tasks at a CAP certification body, and regular verification must be carried out.
In addition, what makes the CAP different from ISO is the biannual verification that takes place. During this verification, the actual specimens are sent by the CAP governing body to each facility to verify that the tests are consistent across CAP facilities.
 中文
中文